Data providers and down-stream analysis
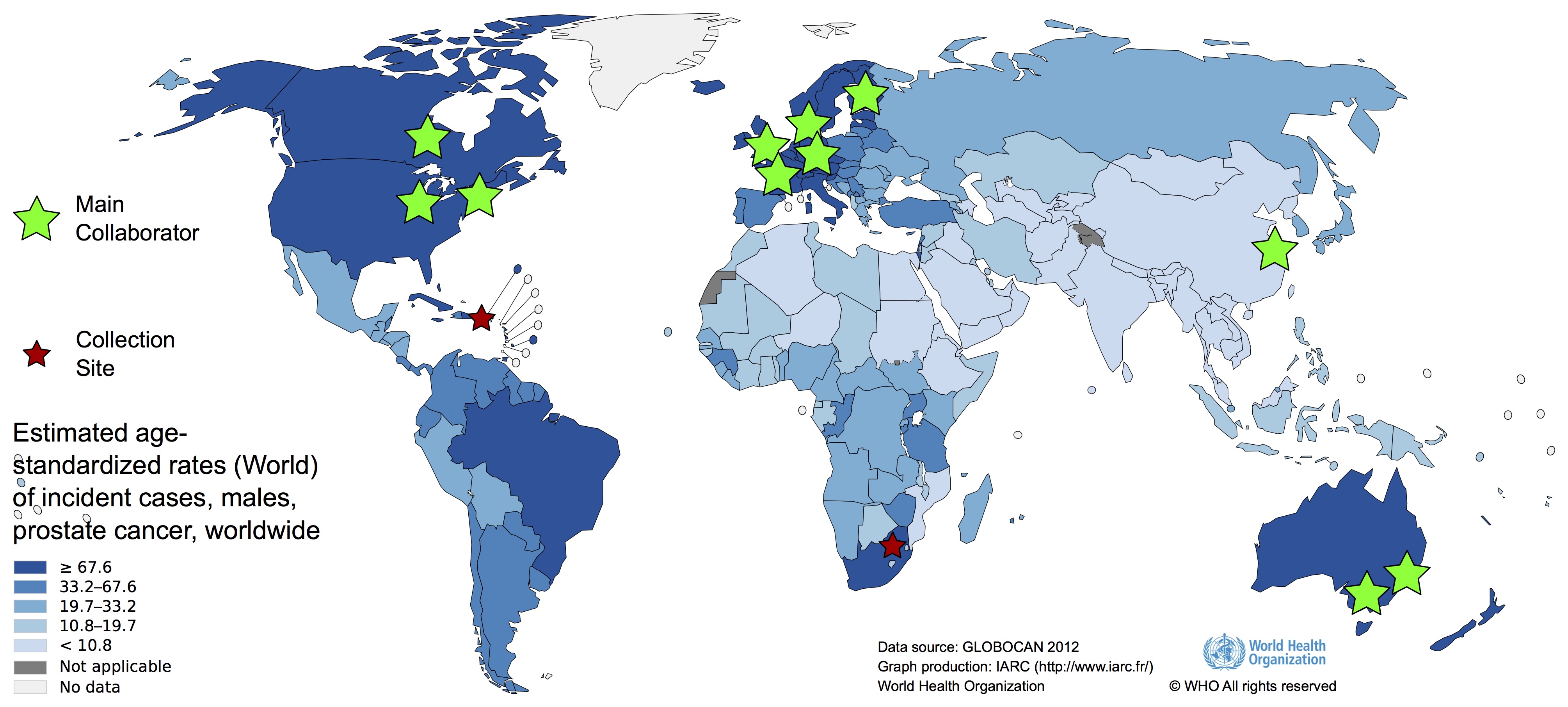
The Melbourne, Australian research group
The Melbourne, Australian research group is focused on three main goals:
- Deciphering the genomic drivers of metastatic potential in prostate cancer
- Tracking the dissemination of cancer cells from the primary organ to distant sites
- Understanding treatment resistance through neoadjuvant clinical studies
Nearly all deaths from cancer are due to primary tumour cells spreading to secondary sites: metastasis. Yet we still do not fully understand why some tumour cells metastasise while others do not. Our work over the past five years has placed our group in a pivotal position to decipher the genomic codes that drive metastasis and hence lethal disease in prostate cancer. We have established a team of researchers with unique expertise in tracking, sampling, and interrogating (with deep genomic analyses) lethal prostate cancer specimens in living patients. Analysing such living specimens is a clinical and technical challenge, but it provides invaluable insights beyond what other approaches can tell us because we can track actual disease spread sequentially in living patients....more...
These analyses in combination with the work from our PPCG co members Steve Bova and David Wedge have revealed the precise patterns and direction of lethal metastatic spread, as well as determined the tumour complexity at primary and metastatic sites. The analysis of mutations specifically associated with metastasis has revealed distinct genomic signatures associated with those tumour cells that have the potential to spread and colonise distant sites [1,2].
Our next crucial step is to map the trajectory of these distinct metastatic tumour cells in a much larger cohort of living patients. These genomic signatures can be used to identify metastatic tumour clusters in the primary tumour, and then we can sample and track these metastatic cells in the blood stream of patients as the disease progresses. Metastasising cancer is so lethal partly because it is difficult to know where and when it will spread throughout the body: we can target the primary tumour because it is localised (in situ), but we struggle to do anything about secondary tumours because they have spread systemically throughout the body and can potentially colonise dozens of different sites. A method for understanding and hence predicting the spread of tumour cells could be a powerful tool for early and effective treatment (or even prevention) of secondary tumours.
Our group will then sample and monitor the dynamics of these tumour cells as they colonise and expand in metastatic lesions in the same patients. We can then link these dynamic clinical outcomes – spread, colonisation, and growth of actual secondary tumour cells (i.e. in living patients) – with the distinct metastatic genomic signatures we have identified. This link will allow us to directly assess the prognostic potential of these metastatic signatures, potentially having a transformative effect on clinical prognosis in prostate cancer, thus enabling clinicians to provide the most appropriate treatments informed by accurate prognosis predictions.
- Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520(7547):353-7.
- Hong MK, Macintyre G, Wedge DC, Van Loo P, Patel K, Lunke S, et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat Commun. 2015;6:6605.
Principal Investigator: Chris Hovens, Niall Corcoran, Tony Costello
Canadian Prostate Cancer Genome Network (CPC-GENE)
The Canadian Prostate Cancer Genome Network (CPC-GENE) is an clinically-driven initiative to identify novel prognostic and predictive molecular biomarkers of clinical outcome for men with intermediate-risk prostate cancer. CPC-GENE is composed of two clinical cohorts: ~300 men who were treated with radical prostatectomy and, uniquely, ~150 men treatment with modern-era, dose-escalated image-guided radiotherapy. All men had at least 5 years of clinical follow-up at the time of enrolment (current median > 9.5 years). Fresh-frozen tumour tissue specimens were obtained for each patient, including a unique set of fresh-frozen tumour biopsies obtained prior to initiation of radiotherapy. Each participant also provided a whole-blood specimen at time of enrolment, for analysis of germline molecular features. ...more...
Tumour and blood-derived genomic DNA was analysed by whole-genome sequencing (60X and 40X target coverage, respectively) to identify single-nucleotide variants (SNVs) and structural variants (SVs), supplemented with array-based profiling of genome-wide DNA copy number aberrations (CNAs) and aberrant DNA methylation. In addition, the tumour transcriptome was profiled using deep RNA-seq (300 million read-pairs) and/or cDNA microarray. Finally, the core CPC-GENE dataset and tissue repository has been leveraged for additional studies of the tumour proteome and epigenome.
CPC-GENE has also profiled intra-prostatic heterogeneity, using multiple tumour foci taken from a subset of men in the main study.
The major findings of CPC-GENE, to date, are
- Saturating discovery of recurrent coding SNVs (i.e. >1%) in localized prostate cancer
- Identification of recurrent non-coding SNVs
- Development and validation of a novel CNA-based prognostic signature of adverse clinical outcome
- Identification of a multi-parametric clinico-molecular signature of rapid biochemical recurrence
- Characterization of the comparative genomics of sporadic localized prostate cancer to familial prostate cancer in men who carry a deleterious germline BRCA2 mutation
- Assessment and identification of prognostic variants within the tumour mitochondrial genome
- Identification of MYCL1 as a candidate oncogene in prostate cancer
- Characterization of a novel mechanism of tumour suppressor silencing involving inversion of the PTEN locus
- Development of novel bioinformatics tools to assess localized hypermutation (ShatterProof) and robustness of whole-genome sequencing data (SeqControl)
Principal Investigators: Michael Fraser, Juri Reimand
Co-Investigators: Theo van der Kwast, and Colin Collins
CRUK-ICGC Prostate Cancer Group
This Cancer Research UK funded Prostate Cancer Group consists of a multidisciplinary team of histopathologists, urologists, molecular biologists, geneticists and experts in genome technology and bioinformatics. We have collected and analyzed 30-50 fold coverage whole genome paired-end sequence data from 250 prostate cancers representing the following groups: (i) cancers from men at different risks of progression; (ii) metastatic castration resistant disease; (iii) apparently separate and genetically distinct (multi-focal) cancers arising in a single prostate; (iii) cancer from arising in men from different ethnic groups. ...more...
In parallel it is our intention to collect transcriptomic and epigenetic data on cancers entered into this study. Analyses of the combined datasets will inform on our understanding of the molecular basis of the genetic and clinical heterogeneity of prostate cancer and will provide insights into the reasons for variation in incidence in different ethnic groups. The results may suggest etiological causes for cancer development, and help to personalized treatment for men who develop prostate cancer.
Joint Lead of Project: Colin S. Cooper and Ros A. Eeles
Principal Investigators: G. Steven Bova, Daniel S. Brewer, Peter Campbell, Christopher S. Foster, Andy G. Lynch, Charlie Massie, David C. Wedge
Danish Prostate Cancer Group
Closely aligned with the German ICGC Prostate Cancer Group. Focused on mutational disease mechanisms and clonal evolution of prostate cancer.
Principal Investigators: Joachim Weischenfeldt
Danish Aarhus University Hospital Prostate Cancer Group
Principal Investigators: Karina Sorensen
Finnish Prostate Cancer Group
Closely aligned with the CRUK-ICGC Prostate Cancer Group. Our collaborative work is focused on uncovering features of lethal prostate cancer useful for improving patient care, through deep integrated clinical-molecular study of PELICAN metastatic prostate cancer rapid autopsy study cases in collaboration Tapio Visakorpi from the University of Tampere, and William B. Isaacs from Johns Hopkins University.
Principal Investigators: G. Steven Bova
French ICGC Prostate Cancer Group
Every year about 70,000 new cases of prostate cancer are diagnosed in France and 8,700 men died from this disease. But the incidence and the rate of mortality per 100,000 men in the French West Indies are respectively 1.5x and 2x upper than the rates observed in the metropolitan France. The aim of this project is to identify, on French Caucasian and African-Caribbean men, relevant genomic events involved in prostate carcinogenesis. The project aims to identify, through an integrative approach, genomic factors which characterize or allow targeting the various phenotypes of aggressiveness of early stages of prostate cancer. The project hypothesizes that molecular events leading to aggressive (clinical progression to extraprostatic disease and hormone refractory disease) are present at the earliest stages of the disease and are modulated by microenvironment and genetic background. ...more...
We propose a multiple stages study design involving firstly the generation of a primary set of genome-wide genomic data (whole genome sequencing, whole exome sequencing, RNA and miRNA sequencing) on 25 tumors of aggressive (high grade) prostate cancer samples (from 15 French Caucasian and 10 African-Caribbean men who were treated by radical prostatectomy). Among the 25 prostate cancer samples, ten will be also selected for methylation analyses. The discovery data will be analysed to catalogue differences that are observed between tumour and referent DNA. We will identify target regions (e.g. somatic mutations, structural variants, and/or singular expression patterns clustering in particular genes, genomic regions or functional pathways) for next stages of the study. Secondly, we plan to validate the clinical relevance of target regions identified on different (5) and large independent sets (500) of prostate cancers specimens according the following phenotypes stratification: (i) Caucasian versus African-Caribbean; (ii) Low grade versus high grade. (iii) Early onset versus late onset; (iv) Free survival disease versus recurrent disease; (v) Metastatic versus non metastatic disease.
Principal Investigator: Olivier Cussenot
Sydney-African prostate cancer research group
Prostate cancer is characterised by significant geographic and ethnic disparity. While incidence rates are highest in Australia, mortality rates are highest in Sub-Saharan Africa. Globally, being from Africa doubles your risk for a prostate cancer associated death. In the United States, being of African ancestry not only doubles your risk, but increases it 3-fold for African American men younger than 65 years of age. This significant health disparity suggesting environment, lifestyle or genetic contributing factors. Our research at the University of Sydney is focused on using cutting-edge genomic technologies and computational big data science to identify these factors. Our vision, to reduce the global burden of prostate cancer associated mortality, while closing the gap to African inclusion in precision health....more...
Together with our partners at the University of Pretoria in South Africa, we established the Southern African Prostate Cancer Study (SAPCS), and with our partners at the University of Nairobi, we have established the East African Prostate Cancer Study (EAPCS) and as such have created an African-specific resource for patient and tumour genome interrogation. Through funding from the Australian National Health and Medical Research Council (NHMRC), we have performed deep whole genome sequencing (blood >40X, tumour >90X) of almost 200 patients, allowing for direct comparison between Southern African patients and clinicopathologically matched tumours from Australian patients from across Sydney. To the best of our knowledge, this is the largest African-derived prostate cancer genomic resource. Identifying a greater tumour mutational burden, as well as African-specific oncogenic drivers and mutational signatures or taxonomies, our data suggesting that an external force/s may be contributing, at least in part, to aggressive disease presentation in Africa.

Principal Investigator: Vanessa M. Hayes
Germany ICGC Prostate Cancer Group - Early Onset
Prostate cancer is the most frequent malignant tumor in males and the second most frequent cause of cancer-related death. Currently, in Germany, more than 60,000 prostate cancers are diagnosed every year. Although most of these patients are treated in a curative attempt, more than 10,000 German men die from prostate cancer annually. Owing to the demographic changes of our society, a further doubling of prostate cancer incidences during the next 20 years is expected. Prostate cancer is generally considered a tumor of elderly men. However, a fraction of prostate cancers are diagnosed at the age of 55 years or less. For several reasons, these “early onset prostate cancers” may represent a key entity for the understanding of prostate cancer biology. ...more...
First, it is likely that early onset prostate cancers represent a distinct molecular subgroup of prostate cancer (PCa), potentially characterized by relatively small numbers of genetic changes, some of which may be particularly strong driver mutations for PCa development. Second, a fraction of prostate cancers in young individuals could represent classical prostate cancers that are detected at a very early stage and might therefore have accumulated molecular changes/mutations occurring that are most instrumental for prostate cancer early detection. Third, PCa with hereditary backgrounds are likely to accumulate in the age group below 55 years. A comparison with other sample sets (e.g. from other ICGC consortia), a systematic genomic analysis of young men with PCa could therefore lead to the detection of mechanisms for hereditary PCa. Fourth, a better understanding of these tumors is particularly relevant as finding optimal treatment regimens is most critical in young cancer patients.
We are analyzing the entire genomic DNA sequences of at least 200 PCas (and matched non-tumorous DNA) of young men (≤ 55y), to at least 30 fold coverage, and integrate single-nucleotide variants and genomic structural variations with differential methylation, mRNA and miRNA expression data.
Tissues were collected at the University Medical Center Hamburg-Eppendorf. Sequencing was performed at DKFZ and NCT (Heidelberg), EMBL (Heidelberg), and MPIMG (Berlin). Data management and bioinformatic data analyses is being conducted at DKFZ.
Head of Consortium: Christoph Plass and Guido Sauter
Principal Investigators: Thorsten Schlomm, Jan Korbel, Benedikt Brors, Marie-Laure Yaspo, Holger Sültmann, Joachim Weischenfeldt
TCGA Prostate Cancer Group
Chris Sander and Massimo Loda co-lead the prostate cancer effort for TCGA, in which molecular analysis of 333 primary prostate carcinomas revealed substantial heterogeneity and major subtypes among patients, as well as potentially actionable lesions valuable for clinical management of the disease (Schultz et al, Cell, 2015). ...more...
The National Cancer Institute and the National Human Genome Research Institute launched The Cancer Genome Atlas program to create a comprehensive atlas of the genomic changes involved in more than 20 common types of cancer. This large-scale, high-throughput effort is being carried out by a network of more than 100 researchers at many organizations across the United States. The overarching goal of TCGA is to further scientific understanding of the genomic changes in cancer, thereby improving the ability to diagnose, treat and prevent this devastating disease. All data generated by the TCGA research network are made rapidly available to the research community through the TCGA Data Portal.
Massimo Loda's group focusses on the following research areas:
- The role of de novo lipogenesis in the initiation and maintenance of prostate cancer.
- The role of the microenvironment in tumor progression in prostate cancer.
- Metabolic profiling in serum and archival tissues as a predictor of clinical behavior and oncogenic drivers.
Principal Investigators: Chris Sander and Massimo Loda
WEHI Computational Cancer Biology and Bioinformatics Group
The WEHI Computational Cancer Biology and Bioinformatics Group develops and applies mathematical, statistical and computational methods to drive discoveries in cancer multi-omics data, with particular interests in structural variant detection and complex genomic rearrangements, modelling and evolution, as well as transcriptional heterogeneity and the tumour micro-environment ...more...
The group works closely with Chris Hovens and Niall Corcoran from the Melbourne Research Group, and is part of the Combimets project. Tony Papenfuss (co-chair), Stefano Mangiola, Ramyar Molania are members of the PPCG RNA working group. Tony Papenfuss and Justin Bedo are members of the PPCG Subtyping working group.
Prinipal investigator: Tony Papenfuss
Co-investigators: Justin Bedo, Stefano Mangiola, Ramyar Molania
Infrastructure providers
DKFZ ITCF Cloud
The German Cancer Research Center (DKFZ) hosts the sequencing data contributed to the PPCG project by Germany. Primary data processing of the German prostate cancer cohort is performed by the division of Applied Bioinformatics at the DKFZ. The research center's IT core facility provides the compute infrastructure to run bioinformatics workflows and to store the results. The compute cluster comprises 504 CPU cores, 9.3 TB of RAM, and up to 10 PB of storage. It builds on OpenStack, an open-source cloud management platform.
Embassy Cloud
Ever-larger scientific datasets may be a goldmine for discovery, but analysing them has become a bottleneck in life-science research. Organisations of all sizes face the challenge of providing infrastructure for managing Big Data, even when the demand for it is comparatively modest.
Embassy Cloud users (tenants) have direct access to the EMBL-EBI data, services and compute. This is a practical and cost-effective alternative to replicating services and downloading vast, public datasets locally. Tenants can access their workspace from anywhere in the world, reducing the need for capital investments in hardware and related operational costs.
Contact: Steven Newhouse
European Genome-phenome Archive
The European Genome-phenome Archive (EGA) is a service for the permanent archiving and sharing of all types of personally identifiable genetic and phenotypic data which are consented for research use. The EGA provides the necessary security required to control access and maintain participant confidentiality, while providing access to those researchers and clinicians authorised to view the data. In 2017 EGA released 1.7 PB of data, resulting in a total of over 5 PB available for authorised users, and distributed over 4.5 PB of data to users. With its background in securely managing large volumes of human genetic and phenotypic data, the EGA is working with the Pan Prostate Cancer Group to provide data co-ordination, archival, and data distribution services.
Principle Investigator: Thomas Keane
Data Co-ordinator: Giselle Kerry
Gilles Thomas bioinformatics facility
The French ICGC prostate group contributed to the PanProstate project by analysing the 25 French samples with the PanProstate standardised pipelines (alignment, variant calling). The sequenced samples were processed in Lyon, on the "Gilles Thomas" bioinformatics facility. This platform was founded in 2009 by Prof. Gilles Thomas in order to provide bioinformatics support to French and international cancer research projects, focusing on both basic and clinical research. This mission is carried out through several international research programmes, in particular the International Cancer Genome Consortium and the French Prostate project, under the supervision of Institut National du Cancer and INSERM.
Contact: contact-pgt@lyon.unicancer.fr
URL: http://www.synergielyoncancer.com/our-projects/bioinformatics-center
Melbourne Bioinformatics
Melbourne Bioinformatics provides computational resources and analytics expertise for the Australian component of the Pan Prostate project, working in close collaboration with Prof Chris Hovens.
Contact: Bernie Pope
URL: https://www.melbournebioinformatics.org.au/project/ppgc/
Wellcome Trust Sanger Institute
Contact: Adam Butler